Radiation
In light of the events at the Fukushima‑daishi nuclear plant I had to go revisit my notes on nuclear physics to make sense of the news. I thought I’d share my notes here in case they prove useful to somebody else. Dear physicist friends, please critique away.
Just what exactly is radiation?
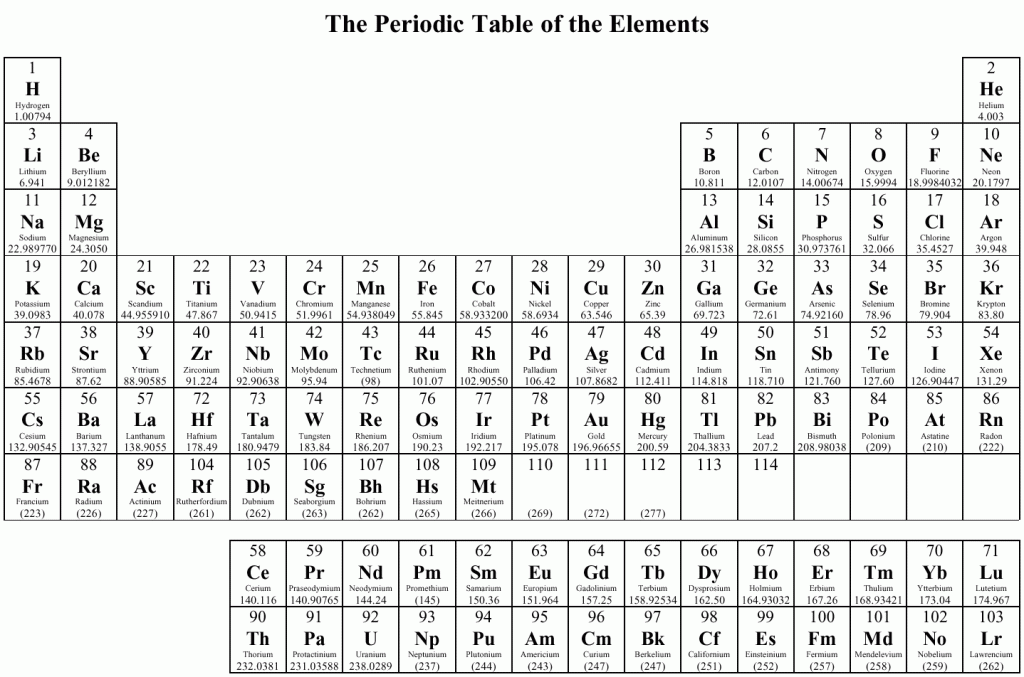
Let’s start with the classic model of the atom: we have a nucleus composed of protons and neutrons and a cloud of electrons surrounding the nucleus. The charge of the atom is balanced because the number of protons is the same as the number of electrons. How many protons the nucleus has determines the kind of element you have.
See those numbers at the top of each box? That’s the number of protons in the nucleus. Note however that since neutrons have no charge, their number can vary and the atom’s electric charge will still be balanced. Chemically, it will behave the same. But atomically it is different. These atoms that have a different number of neutrons in the nucleus are called isotopes.
You might recall that electrically, like charges repel and different charges attract. Since all protons are positively charged, why doesn’t the nucleus disintegrate? The answer has to do with a force much more powerful than the electromagnetic force that makes protons repel each other, but that acts only over very short distances, the strong‑nuclear force.
All those neutrons inside the nucleus act like a kind of cement, binding the protons together. Also the protons both attract each other (strong nuclear force) and repel each other (electromagnetic force) and both forces diminish rapidly the farther apart the protons are. However, the strong nuclear force diminishes much, much faster than the electromagnetic force. Move the protons too far apart and the strong nuclear force starts losing out to the electromagnetic force. That is exactly what happens when you have too many neutrons in the nucleus. They push the protons farther apart, to the point that the strong nuclear force is not enough to keep the nucleus together since the electromagnetic force is pushing the protons apart. This is a long way of saying that most nuclei with too many neutrons turn out to not be very stable.
So what happens with an unstable isotope? It emits particles from the nucleus to stabilize itself. These particles can be one of two kinds:
-
Two protons and two neutrons (a Helium nucleus) shoot out from the nucleus of the isotope. This is called an Alpha particle, and it has a positive charge (two protons and no electrons).
-
A neutron emits an electron (the neutron turns into a proton) or a proton emits an anti‑electron (positron) and turns into a neutron. These electrons or positrons emitted from the nucleus are what are called beta particles.
Once an unstable nucleus has emitted alpha or beta particles, it remains in an excited state (at a higher than normal energy level), but this doesn’t last very long. What happens is that this extra energy is released in the form of gamma rays, which are very high energy photons.
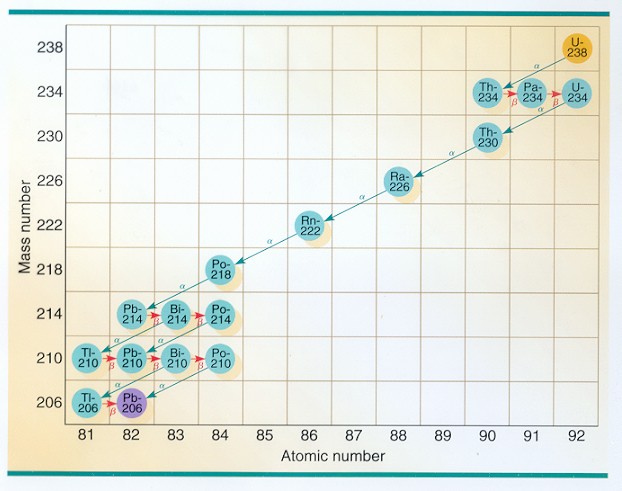
And what happens to the nucleus? Well, since each alpha particle emitted makes it lose two protons, it drops by two squares in the periodic table. Uranium‑238 becomes Thorium‑234 by emitting an alpha particle and some gamma rays. And since each beta emission either turns a neutron into a proton or viceversa, the element can “decay up” to the next element in the periodic table or “decay down” to the previous element. Thorium‑234 becomes Protactinium‑234 by the emission of a negatively charged beta particle. Here is the full decay chart of Uranium‑238.
So what is radioactivity? It is the emission of alpha and beta particles plus some gamma rays from unstable isotopes that are decaying into more stable isotopes.
Now, we don’t know exactly when a particular nucleus will decay, it has about equal probability of decaying or not decaying at any particular time (Hi Schrödinger!), but we do know that if we have a certain amount of material, eventually enough of the nuclei will decay that we are going to be left with about half of the original material and half of whatever it decays to. How long will this process take? It depends on the specific isotope, but this is what is called the half‑life or the isotope.
Why is Radiation Dangerous?
You might have heard the term 'ionizing radiation'. What is ionizing radiation? Well, remember those alpha particles, beta particles, and gamma rays? They have a lot of energy. In fact, they have so much energy that if they collide with another atom, they can easily knock out an electron from it. This leaves the atom positively charged or 'ionized'. The problem with ionized atoms is that since they are no longer electrically neutral, they tend to combine with other atoms forming ionic bonds.
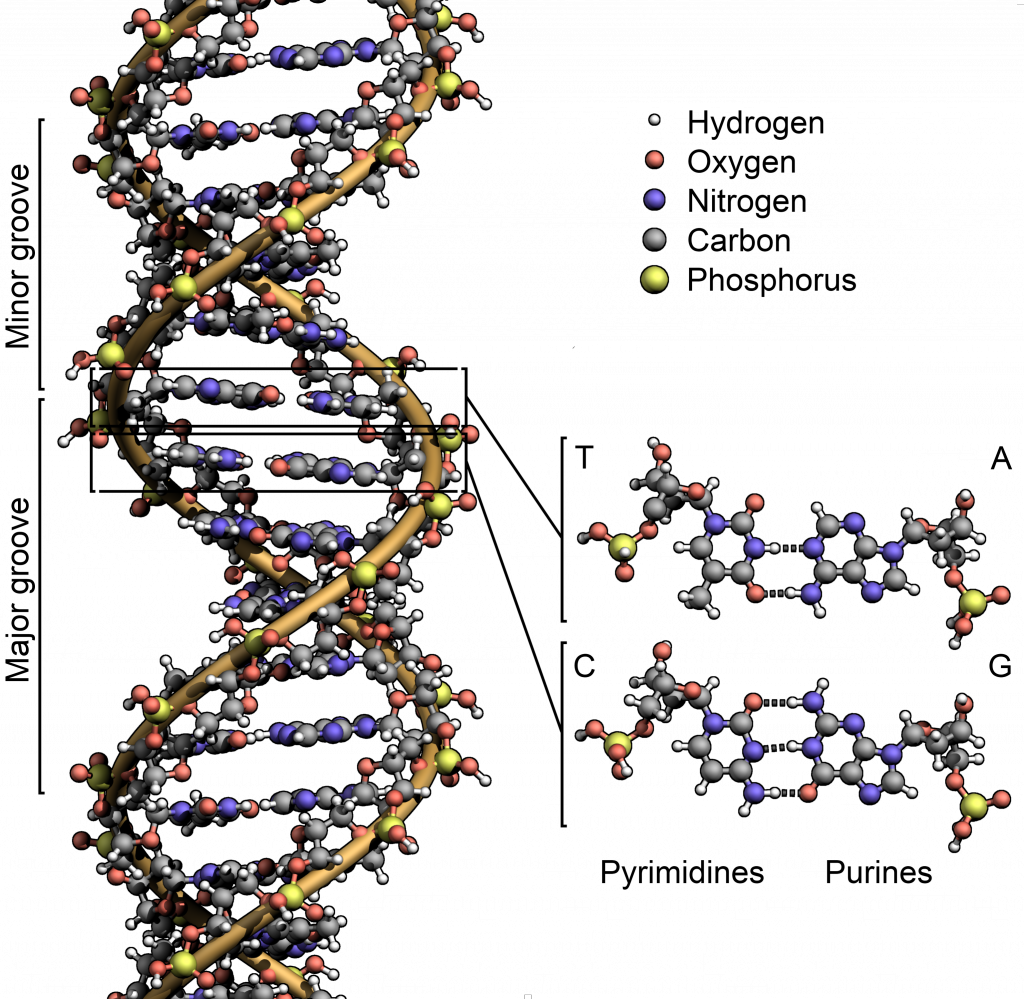
So what does ionizing radiation do to the body? Well, DNA’s amino acids are mostly composed of Hydrogen, Oxygen, Nitrogen, Carbon, and Phosphorous. If one of these lose particles goes knocking off electrons from atoms, the electrical properties of said atoms change, altering the chemical properties of the molecules they belong to, which can damage the DNA.
Damaged DNA can result on one of three outcomes:
-
The injured cell can repair itself, resulting in no residual damage.
-
The injured cell dies. Not much different than millions of cells that die and get replenished every day.
-
The injured cell incorrectly repairs itself, resulting in a mutation.
It’s this third case that we are generally worried about.
Now, remember that in order to damage the cell, a given particle needs to actually hit it. Let’s take a look at the particles and have some idea of their energy levels.
Alpha particles
Remember that these are Helium nucleus, so two protons and two neutrons. What this means is that they are big (compared to beta particles which are electrons and gamma rays which are photons). The energy an alpha particle carries depends on the kind of isotope that emitted it. The heavier elements emit higher energy alpha particles. In general they have between 3 and 7 MeV (Mega‑electron‑volts). Even though this is a lot of energy for a single particle, they are also pretty massive (a proton has roughly 2,000 times the mass of an electron) so they don’t go that fast. They are also positively charged, which means they get deflected by magnetic fields.
Because of all these reasons, alpha particles are not particularly dangerous unless you ingest the isotope producing them. If you just happen to be around them, they won’t even penetrate your skin, and they can be blocked using a sheet of paper (so much for an alpha‑particle death ray).
However, if you do ingest them, they are pretty nasty as alpha radiation is one of the most destructive forms of ionizing radiation. The damage it can cause is calculated to be between 10 to 1000 greater than beta or gamma radiation. One example of this is Polonium‑210, which is typically found in cigarettes. You smoke it, it generates alpha radiation inside your lungs, you get cancer. That’s how they whacked Alexander Litvinenko, they poisoned him with Polonium‑210.
Beta Particles
Beta particles are much less massive than alpha particles, so they are about 100 times more penetrating. Beta radiation can be blocked using a piece of aluminum (get out the tinfoil hats!). They are typically used in medicine precisely because they penetrate the human body so well. For instance, PET scans use a radioactive tracer isotope as a source of positrons. So yeah, you get irradiated with them for medical reasons.
Gamma Rays
Gamma rays are a bit nastier than both alpha and beta particles because they are electrically neutral, they are much more penetrating (what stops a particle other than colliding is the electromagnetic fields). They tend to cause more cell damage when they have about the same energy as an alpha particle (between 3 and 5 MeV).
Measuring Radiation
So how do we measure radiation? This is where the story gets complicated. Radiation is measured using a mishmash of different units, depending on what exactly you are measuring.
At the most basic level, you measure radioactivity. Namely the amount of ionizing radiation released by a material. This measure doesn’tmake a distinction between alpha or beta particles or gamma rays. It just measures how many nuclei are decaying per unit of time (usually seconds). The old unit of measurement was the Curie (Ci) and the new one is the Becquerel (Bq). One Curie is equal to 37 billion (3.7 x10^10) disintegrations per second. One Becquerel is one disintegration per second. It follows that one Curie is equal to 37 billion Becquerels.
The other measurement is exposure: the amount of radiation traveling through the air. The units of exposure are the Roentgen ( R) and Coulomb/Kilogram (C/kg). One Roentgen is the amount of radiation necessary to produce a charge of 0.000258 C/kg under standard conditions.
The absorbed dose is the amount of radiation absorbed by an object. I.e. the amount of energy deposited in the object through which the radiation passed. The units are radiation absorbed dose (rad) and gray (Gy). An absorbed dose of 1 rad means that 1 gram of material absorbed 100 ergs of energy. One gray is equivalent to 100 rad.
Finally, the effective dose combines the absorbed dose and the medical effects of abosorbing that type of radiation. This is because living cells respond different to alpha particles than to beta particles or gamma radiation. Alpha particles tend to be more damaging than beta particles or gamma rays. The units for the effective dose are the Roentgen equivalent man (rem) and the Sievert. The rem is just the roentgen adjusted by the amount of damage the specific type of radiation does the to body. For instance, the weighting factor for beta particles and gamma rays is one. For alpha particles it is 20. The Sievert is equivalent to 100 rem.
For healt and risk purposes, the only measure we care about is the Sievert (or rem). Getting sprayed with alpha particles is much more damaging than getting sprayed with beta particles (in fact, 20 times more damaging).
On average we receive a dosis of 0.62 rem (6.2 mSv) every year. About half of it comes from natural background radiation, and the other half from man‑made sources (X‑Rays for instance).
Randall Munroe has helpfully created a chart of typical amounts of the effective dose of radiation measured in Sieverts. I suggest you take a look.
Radioactive Fallout
Inside a nuclear reactor, there are all sorts of isotopes being created and decaying. The ones you will mostly read about are Iodine‑131, Caesium‑137, and Strontium‑90.
Iodine‑131
This isotope has been on the news as of lately. Its half‑life is just about 8 days, and it typically decays by emitting beta particles and gamma rays. It tends to enter the food chain and if you eat it it accumulates in the thyroid gland. That’s why they give you iodine tablets for radiation sickness, not because it does anything to stop the radiation, but because it saturates your body with non‑radioactive iodine so your thyroid gland doesn’t accumulate the radioactive one.
Caesium‑137
Caesium‑137 has a half‑life of about 30 years, so it is more problematic than Iodine‑131 which decays rapidly. However, once it has entered the human body, it gets uniformly distributed and has a biological half‑life of about 70 days. Like Iodine‑131 it decays by emitting beta and gamma radiation.
Strontium‑90
Strontium‑90 has a half‑life of about 28 years and it is normally referred to as a “bone seeker”. This is because it is biochemically similar to calcium, so after entering the organism, about 30% of it gets accumulated in the bones and bone marrow. The rest gets excreted.
 Oscar Bonilla
Oscar Bonilla